

For example, a quick glance at Figure AT5.2. In chemistry, reactivity is a measure of how readily a substance undergoes a chemical reaction. That way, we can get a better look at the relationship. Often it is useful to plot data on a graph. As a result, fluorine is usually thought of as the most electronegative element. However, on many scales, fluorine would be the most electronegative atom here. As a result, noble gases are also given electronegativity values on this scale. The Periodic Table of the Elements is an additional interactive that has information and background on all of the known elements, linked to relevant sections in the text. The Allen scale just depends on the ability of an atom to interact with light, which is something even noble gases can do. Skip navigation 0:00 / 3:46 Which Is The Most Reactive Element In The Periodic Table ScienceABC II 2.88K subscribers Subscribe 902 views 3 months ago reactivity periodictable cesium The. Some electronegativity scales do not have values for the noble gases, because they are based on experimental measurements of compounds, and noble gases do not commonly form compounds with other elements. It's true today as it was in Mendeleev's time.

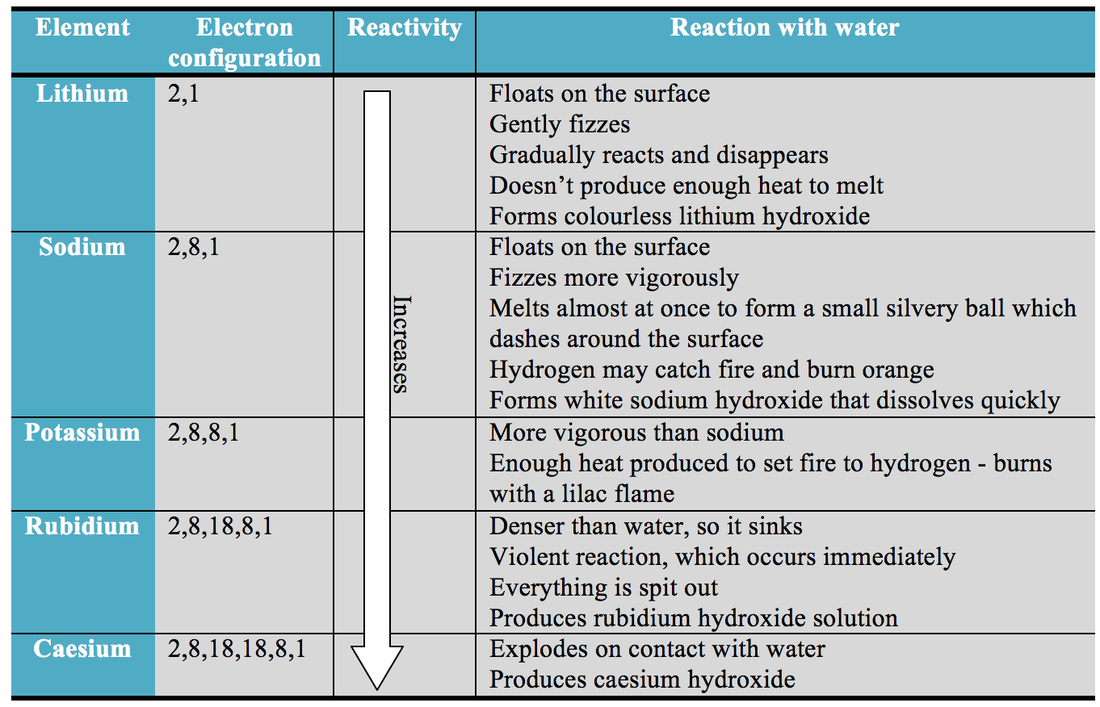
Metals towards the top of the chart are highly reactive and metals towards the bottom, such as gold, are. \): The Allen electronegativity values of the second-row elements. The table gets its shape from the properties of the elements, like relative weight, conductivity, and reactivity. The relative reactivity of different metals can be seen on the reactivity of metals chart.


 0 kommentar(er)
0 kommentar(er)
